 On Friday, Jan-Lukas Førde has defended his PhD work entitled “The Zebrafish larva as a model system for myeloid malignancies”. He has worked to develop the zebrafish larva as a tool to investigate new therapises for myeloid leukaemia. First, by writing a new software for high-content analysis of fluorescent objects in zebrafish larvae. Then he used the software to find the efficacy of a new drug for AML in transplanted larva. Finally, he studied how a drug carrier influenced macrophages in zebrafish larvae. The work was praised by the opponents for the high technical level of the experiments. We are all very proud of Jan-Lukas and his research. Our gift to him? Art depicting zebrafish larva development!
On Friday, Jan-Lukas Førde has defended his PhD work entitled “The Zebrafish larva as a model system for myeloid malignancies”. He has worked to develop the zebrafish larva as a tool to investigate new therapises for myeloid leukaemia. First, by writing a new software for high-content analysis of fluorescent objects in zebrafish larvae. Then he used the software to find the efficacy of a new drug for AML in transplanted larva. Finally, he studied how a drug carrier influenced macrophages in zebrafish larvae. The work was praised by the opponents for the high technical level of the experiments. We are all very proud of Jan-Lukas and his research. Our gift to him? Art depicting zebrafish larva development!
Category Archives: Uncategorized
New drug for leukaemia tested in zebrafish larvae

Acute myeloid leukaemia (AML) is a deadly disease with few treatment options compared to many other cancers. We have tested a molecule, the RAC-inhibitor EHop-016, which inhibits migration of cancer cells. We tested the effect of this molecule on AML cells transplanted into zebrafish larvae, and found that the cells did not migrate to the haematopoietic tissue. More importantly, we found that treatment with EHop-016 together with the commonly used AML drug daunorubicin was better that either drug alone, and could be a way to overcome therapy resistance. These findings were recently published in the journal Translational Oncology.
Biological drugs activate the complement system

We have investigated to which extent biopharmaceutics activate the complement system. This is important, since the complement system is designed to mark unwanted components like viruses in our blood, for degradation. If a drug activates the complement system, it will be eliminated faster, but it can also trigger other immune-related events. This work was in collaboration with Profs. Silje Skrede at Dept. Clin. Sci, Tom Eirik Mollnes at Nordland Hospital, and Kjell-Morten Myhr and Øyvind Thorkildsen at Neuro-SysMed, University of Bergen. The work was recently published in International Immunopharmacology.
Can zebrafish larvae get human leukaemia?
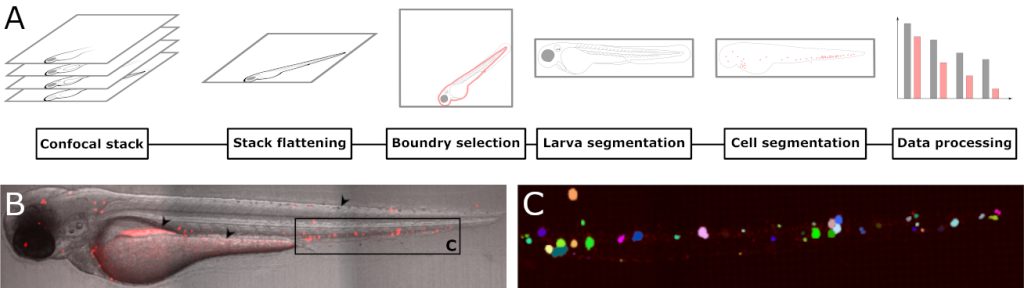
We have been working with zebrafish larvae as a model system for human diseases for a while, and have recently showed that they are excellent systems to study development of leukaemia. Fluorescently labelled leukaemia cells can be transplanted into the larvae by intravenous injection. In a recently published paper, we have written a software plug-in which automatically counts the number of individual leukaemia cells based on confocal microscopy images. In this way, we can follow how the leukaemia develops, both over time, and how they distribute in the larvae. Although tailor-made for larvae transplanted with leukaemia cells, the software can be used to analyze any fluorescent particle. This work was recently published in Biology open.
Liposomes designed to protect the heart during cancer therapy
One of the main advantages of using liposomes to encapsulate anti-cancer drugs is that normal tissues like the heart is protected from harmful effects. Still, liposomal drugs are not the first choice partly because they are not more effective in killing cancer cells. We wanted to add further protection of the heart by adding statins in the liposomes along with the anti-cancer drug doxorubicin. Statin protects the hearts from being damaged by doxorubicin, and to our pleasant surprise, also potentiated the anti-cancer effects. We managed to develop a liposome carrying both statins and doxorubicin, and our liposomes had the same protecting effect on the heart, while retaining the increased ability to kill cancer cells. Although both these drugs can be administered in their free form, liposomal encapsulation is a huge advantage, since both drugs will be equally distributed in the body. Then the heart will be protected, and the cancer cells efficiently killed by the presence of both drugs at the same time.
The results was recently published in International Journal of Pharmaceutics.

New formulation for repurposing of drugs

Repurposing, or redefinition of drugs from one disease to another, is a way to improve treatment of diseases with few therapy options. For acute myeloid leukaemia, the anti-psychotic drug chlorpromazine has showed good effect in cell cultures in vitro. However, it has quite dramatic effects on the central nervous system in non-psychotic individuals. PhD student Edvin Tang Gundersen has developed a nanoparticle which encapsulates chlorpromazine, and prevents it from entering the central nervous system. The formulation is efficient towards AML cells and the nanoparticles prevent the encapsulated molecules crossing from the blood into the central nervous system in zebrafish larvae. The work was recently published in International Journal of Pharmaceutics.
New and promising nanocarriers in cancer therapy

Together with Hanne R Hagland and Abdelnour Alhourani at the University of Stavanger, we have investigated if graphene nanosheets can be used to transport small anti-cancer drugs in the blood. Graphene nanosheets have unique properties that are not found in other nanomaterials, such as a very high surface area for absorption of drugs. We found that graphene released the drugs at low pH, which are perfect for cancer drugs, since most tumours have lower pH than the rest of the body. We also demonstrated that pristine graphene is superor to graphene oxide, but that PEGylation is needed to ensure that the nanosheets disperse nicely in aqueous media. The work was just accepted for publication in ACS Omega.
New drugs inspired from the nature
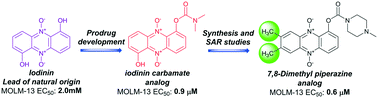
Many years ago, we came across an interesting compound, iodinin, from marine benthic bacteria, which selectively induced apoptosis in leukaemia cells. In fact, the compound was first described more than 70 years ago, but has not been left much attention. We also almost put it aside, until our good collaborators Pål Rongved and Elvar Örn Viktorsson managed to develop a method to synthesise iodinin in the lab, and even better, produce variants which could be even better as drugs. We recently got our work accepted for publication in RCS medicinal chemistry. Here, Pål and Elvar has produced more than 60 analogues of iodinin, which we tested for ability to kill leukaemia cells. The results can tell us something about which parts of the molecules are crucial for the activity, and which part can be improved so we get even better activity, and milder side-effects.
Our own nanocarriers can also transport drugs
Inside our blood, there are several natural nanocarriers. Lipoproteins are small packages of lipids, stabilised by proteins. These transport lipids and some lipid-soluble vitamins in the blood, and deliver them at the target sites. We have found that some lipid-soluble drugs also associate with lipoproteins, and a large fraction of these are transported by lipoproteins rather than serum proteins. This new concept of drug transport in the blood means that we have to think differently when estimating pharmacokinetic properties of some drugs. The paper was published in the journal Therapeutic Drug Monitoring.
New molecules to treat leukemia?
Although immune-based therapies emerge as promising to fight cancers, we will still rely on small molecules for most of the patients. Together with researchers at Clermont-Ferrand, we have investigated inhibitors of Pim-kinases to kill acute myeloid leukaemia cells. Of the molecules we tested, one was particularly promising, and proved efficient also towards leukaemia cells isolated from patients that had developed chemotherapy resistance. Why was our molecule so efficient? Probably because in addition to blocking Pim-kinase activity, it also inhibited other key signalling factors in the leukaemia cells. The work was recently published in Molecular Cancer Therapies.
 The figure shows the different molecules tested, and VS-II-173 stands out being very efficient towards acute myeloid leukaemia (AML) cells, and not towards non-malignant cells. Green indicates low toxicity, red indicates high toxicity.
The figure shows the different molecules tested, and VS-II-173 stands out being very efficient towards acute myeloid leukaemia (AML) cells, and not towards non-malignant cells. Green indicates low toxicity, red indicates high toxicity.